what is odd electron molecule|Exceptions to the Octet Rule : Clark Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in . Billige bus billetter fra København til Århus kan starte fra så lidt som 103 DKK (13 €), når du booker i forvejen. Den gennemsnitlige bus billetpris for København til Århus er 267 DKK (34 €); dog varierer priserne afhængigt af tidspunktet på dagen og klassen, og de plejer at være dyrere på selve dagen.
PH0 · Odd‐Electron Bonds
PH1 · Odd
PH2 · Exceptions to the Octet Rule
PH3 · ChemTeam: VSEPR
PH4 · 9.11: Exceptions to the Octet Rule
PH5 · 6.5: Exceptions to the Octet Rule
PH6 · 10.5: Exceptions to the Octet Rule
What Does It Mean to Have “Action” . and some books will void a bet should a player retire at any point. It Isn’t Limited to Just Tennis and Baseball. Of course, any outdoor sport runs the risk of weather cancellations or games being rescheduled. Extenuating circumstances could force even an indoor match or game to be delayed.
what is odd electron molecule*******There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired electrons. Most odd electron species are highly reactive, which we call Free Radicals. .Odd-electron Molecules. We call molecules that contain an odd number of electrons .
One class of such compounds are those that have an odd number of electrons. As the octet rule requires eight electrons around each atom, a molecule with an odd number . Odd-Electron Molecules. There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in .Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in .Odd-electron Molecules. Molecules that contain an odd number of electrons are called radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when .
VSEPR Structures of Odd Electron Molecules. All the usual rules of building a VSEPR structure will apply - minimize formal charge, build octect on more electronegative ligand .Exceptions to the Octet Rule VSEPR Structures of Odd Electron Molecules. All the usual rules of building a VSEPR structure will apply - minimize formal charge, build octect on more electronegative ligand .
Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule, because the rule requires eight electrons (or two for hydrogen) around each . 1 Introduction. Chemists are familiar with the concepts of even (two, four, six, etc.) electron bonds, but far less so with their odd (one, three) electron equivalents. This is mostly because odd-electron .complexes. 1. Introduction. Chemists are familiar with the concepts of even (two, four, six, etc.) electron bonds, but far less so with their odd (one, three) electron equivalents. .
Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals.Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen .
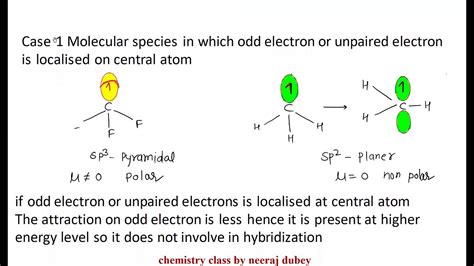
Odd-Electron Molecules. There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide \(\left( \ce{NO_2} \right)\). Each oxygen atom contributes six valence electrons and the nitrogen atom .what is odd electron molecule Exceptions to the Octet Rule Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen .We would like to show you a description here but the site won’t allow us. Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen .
Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five .what is odd electron molecule3. Test molecular ion identity; must be the highest mass peak in spectrum, odd-electron ion, and give logical neutral losses. Check with CI or other soft ionization (TODAY). 4. Mark ‘important’ ions: odd-electron and those of highest abundance, highest mass, and/or highest in a group of peaks (TODAY).
all of the above. Solution. Verified by Toppr. ClO2,N O2,N O : all are odd electron molecules. In these compounds, oxygen have even number of electrons while nitrogen and chlorine atoms have odd number of electrons, making the overall molecule as odd electron molecule. Was this answer helpful? This paper highlights the molecular essence of graphene and presents its hydrogenation from the viewpoint of the odd-electron molecular theory. This chemical transformation was performed computationally, using a particular algorithm, through the stepwise addition of either hydrogen molecules or hydrogen atoms to a pristine .
Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen .Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures.Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when . The molecular orbitals of a given symmetry are numbered in order of increasing energy, for example, 1 σg, 2 σg, 3 σg. Figure 1.11.2: H + 2 molecular orbitals. The lowest-energy orbital, as we have come to expect, is nodeless. It obviously must have cylindrical symmetry ( λ = 0) and inversion symmetry ( g ).
A molecule has 9 valence electrons. What is true about its Lewis structure? (a) It will follow the octet rule. (b) It will be an exception to the octet rule because it is an odd electron molecule. (c) It will be an exception to the octet rule .Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures.Odd-electron molecules represent the first violation to the octet rule. Although they are few, some stable compounds have an odd number of electrons in their valence shells. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2.Steps to Draw Lewis Structures for an Odd Number of Valence Electrons. Step 1: Calculate the total valence electrons. Step 2: Determine the central atom and draw single bonds between the central . 1 Introduction. Chemists are familiar with the concepts of even (two, four, six, etc.) electron bonds, but far less so with their odd (one, three) electron equivalents. This is mostly because odd-electron bonded species are open shell, and therefore usually kinetically less stable under normal conditions than closed-shell molecules.
Notes: If you know your password, but just want to change it, go to account.microsoft.com Security tab, and select Change my password. If you know your username and password but they have stopped working, read My username and password have stopped working.
what is odd electron molecule|Exceptions to the Octet Rule